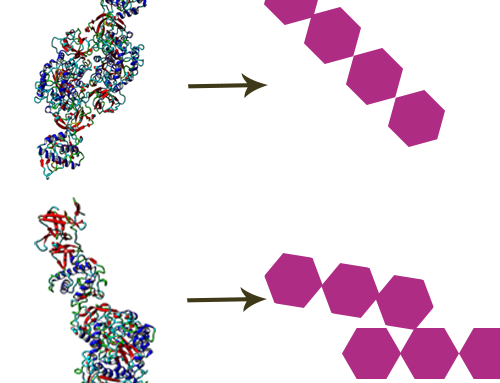
The human digestive system consists of a complex series of organs and glands regulating the processing of food. The process starts in the mouth, where the food breaks down by chewing, and the digestive enzymes in the saliva. Then in the stomach the gastric phase of digestion takes place. At the final stage the food is mixed with enzymes produced by the pancreas, after which the content passes the gastrointestinal tract. The undigested food particles end up in the large intestine, where they are fermented by the gut microbiome, consisting of approximately thousand bacterial species. CarbExplore is an expert in manufacturing, purification and analysis of prebiotic food compounds, such as Fructo-oligosaccharides (FOS), Galacto-oligosaccharides (GOS), Isomalto-oligosaccharides (IMO), Isomalto-/maltopolysaccharides (IMMP), milk oligosaccharides, and N-glycans of milk proteins. These potential prebiotic food components support the growth of the microorganisms that provide a health benefit.
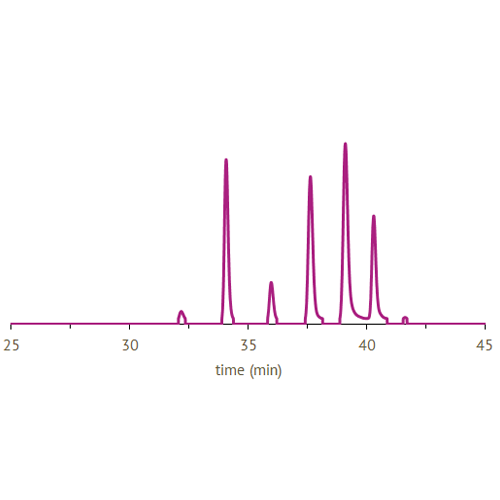
CarbExplore has a library with a broad range of Carbohydrate-Active Enzymes, e.g. glucansucrase enzymes (dextransucrases, mutansucrases, reuteransucrases, alternansucrases), alpha-glucanotransferases, beta-galactosidases and fructansucrase enzymes (inulosucrases, levansucrases). These enzymes are able to convert substrate molecules into the products of interest. We are able to enzymatically modify the carbohydrate structures and functions of the targeted substrates, introducing changes in the degree of polymerization (DP), branching degree, and glycosidic linkages present (α1→2), (α1→3), (α1→4) and (α1→6) (in linear or alternating chains). Our scientists use several techniques (HPLC, HPAEC-PAD, NMR, methylation analysis, MALDI-TOF, HP-SEC) to structurally analyze the products, and to isolate the products of interest.
Examples:
✔ α-Glucan polymers or oligosaccharides with different DP values and linkage types, synthesized from sucrose using glucansucrase wild type and mutant enzymes
✔ Fructan polymers (inulin, levan) or oligosaccharides with different DP values and linkage types, synthesized from sucrose using fructansucrase wild type and mutant enzymes
✔ Mixtures of GOS with different DP values and/or linkage types, synthesized from lactose using β-galactosidase wild type and mutant enzymes
✔ IMO and/or IMMP synthesized from starch (amylose and/or amylopectin) using 4,6-α-glucanotransferase wild type and mutant enzymes
✔ (α1→3)-branched IMO and/or IMMP, using branching sucrase enzymes and sucrose
✔Synthesis, purification and characterization of milk oligosaccharides
✔Large-scale quantitative isolation of pure N-linked glycans of milk proteins

Workflow:
You deliver:
Detailed project information about the product of interest.
We deliver:
A project plan with detailed descriptions.
Our Relevant Publications:
Publications 2020
Gangoiti, J. et al., 2020. Synthesis of novel α-glucans with potential health benefits through controlled glucose release in the human gastrointestinal tract. Critical Reviews in Food Science and Nutrition, 2 1, 60(1), pp. 123-146. DOI 10.1080/10408398.2018.1516621
te Poele, E. et al., 2020. Development of slowly digestible starch derived alpha-glucans with 4,6-α-glucanotransferase and branching sucrase enzymes. Journal of Agricultural and Food Chemistry, 21 5.p. acs.jafc.0c01465. DOI 10.1021/acs.jafc.0c01465
Publications 2019
Pham, H. T., Ten Kate, G. A., Dijkhuizen, L. & Van Leeuwen, S. S., 2019. Synthesis and Characterization of Sialylated Lactose- and Lactulose-Derived Oligosaccharides by Trypanosoma cruzi Trans-sialidase. Journal of Agricultural and Food Chemistry, 27 3, 67(12), pp. 3469-3479. DOI 10.1021/acs.jafc.8b06974
Valk-Weeber, R. L., Dijkhuizen, L. & van Leeuwen, S. S., 2019. Large-scale quantitative isolation of pure protein N-linked glycans. Carbohydrate Research, 1 6, Volume 479, pp. 13-22. DOI 10.1016/j.carres.2019.04.011
Publications 2018
Gangoiti, J., Pijning, T. & Dijkhuizen, L., 2018. Biotechnological potential of novel glycoside hydrolase family 70 enzymes synthesizing α-glucans from starch and sucrose. Biotechnology Advances, 36(1), pp. 196-207. DOI 10.1016/j.biotechadv.2017.11.001
Pham, H., Pijning, T., Dijkhuizen, L. & Van Leeuwen, S. S., 2018. Mutational analysis of the role of the glucansucrase Gtf180-ΔN active site residues in product and linkage specificity with lactose as acceptor substrate. Journal of Agricultural and Food Chemistry, 28 11, 66(47), pp. 12544-12554. DOI 10.1021/acs.jafc.8b04486
Pham, H. T., Dijkhuizen, L. & van Leeuwen, S. S., 2018. Structural characterization of glucosylated GOS derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydrate Research, 1 12, Volume 470, pp. 57-63. DOI 10.1016/j.carres.2018.10.003
van Leeuwen, S. S. et al., 2018. Regional variations in human milk oligosaccharides in Vietnam suggest FucTx activity besides FucT2 and FucT3. Scientific Reports, 1 12.8(16790). DOI 10.1038/s41598-018-34882-x
Yin, H., Dijkhuizen, L. & van Leeuwen, S. S., 2018. Synthesis of galacto-oligosaccharides derived from lactulose by wild-type and mutant β-galactosidase enzymes from Bacillus circulans ATCC 31382. Carbohydrate Research, 30 7, Volume 465, pp. 58-65. DOI 10.1016/j.carres.2018.06.009
Figueroa-Lozano, S. et al., 2018. Dietary N-Glycans from Bovine Lactoferrin and TLR Modulation. Molecular Nutrition and Food Research, 1 1, 62(2), p. 1700389. DOI 10.1002/mnfr.201700389
Publications 2017
Pham, H. T., Dijkhuizen, L. & van Leeuwen, S. S., 2017. Structural characterization of glucosylated lactose derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydrate Research, Volume 449, pp. 59-64. DOI 10.1016/j.carres.2017.07.002
Yin, H. et al., 2017. Engineering of the Bacillus circulans β-galactosidase product specificity. Biochemistry, 7 2, 56(5), pp. 704-711. DOI 10.1021/acs.biochem.7b00032
Publications 2016
Publications 2015
Leemhuis, H. et al., 2014. Isomalto/malto-polysaccharide, a novel soluble dietary fiber made via enzymatic conversion of starch. Journal of Agricultural and Food Chemistry, 10 12, 62(49), pp. 12034-12044. DOI 10.1021/jf503970a
Publications 2014
Wilbrink, M. H. et al., 2014. Galactosyl-lactose sialylation using Trypanosoma cruzi trans-sialidase as the biocatalyst and bovine κ-casein-derived glycomacropeptide asthe donor substrate. Applied and Environmental Microbiology, 80(19), pp. 5984-5991. DOI 10.1128/AEM.01465-14
Publications 2013
Leemhuis, H. et al., 2013. Glucansucrases: Three-dimensional structures, reactions, mechanism, α-glucan analysis and their implications in biotechnology and food applications. Journal of Biotechnology, 1, 163(2), pp. 250-272. DOI 10.1016/j.jbiotec.2012.06.037
Publications 2012
Anwar, M. A. et al., 2012. The role of conserved inulosucrase residues in the reaction and product specificity of Lactobacillus reuteri inulosucrase. FEBS Journal, 10, 279(19), pp. 3612-3621. DOI 10.1111/j.1742-4658.2012.08721.x
Desmet, T. et al., 2012. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chemistry – A European Journal, 27 8, 18(35), pp. 10786-10801. DOI 10.1002/chem.201103069
Publications 2011