Glycosylation is a commonly used strategy to alter the physicochemical properties of compounds. Glucansucrase enzymes efficiently glucosylate a diversity of small molecules. When incubated with only sucrose, glucansucrases synthesize α-glucan poly- and oligosaccharides from sucrose. In the presence of suitable acceptor substrates, virtually only their glucosylation is observed. One example is the Lactobacillus reuteri 180 Gtf180 glucansucrase, α-glucosylating (non-)carbohydrate molecules. Glucosylation of compounds may improve their properties, e.g. resulting in increased solubility or bioavailability, strengthening their efficacy as taste enhancer, or allow synthesis of (mimics of) human milk oligosaccharides.
Enzymatic glycosylation instead of chemical glycosylation has major advantages for sustainable production processes. The glucansucrases are active at body temperature and ambient pressure, and in mildly acidic solutions. They are also solvent resistant, in case solvents are needed to dissolve the potential substrates of interest. Enzymatic glycosylation thus is more environmentally friendly and reduces energy consumption. Moreover, enzymes catalyze very specific types of reactions. Examples of our work are summarized below (also see literature references provided).
Glucosylation of steviol glycosides
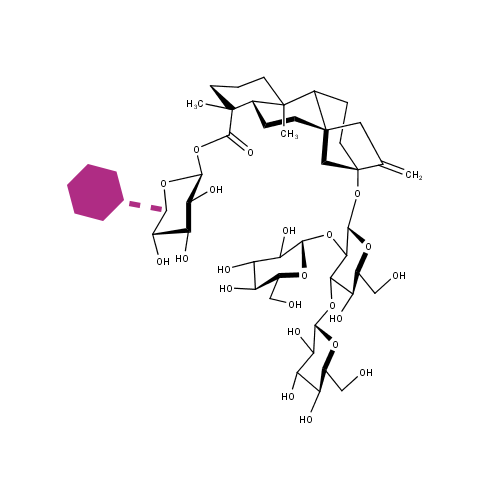
Steviol glycosides are the main active compounds in the leaves of Stevia rebaudiana. These non-caloric compounds are commonly used as natural sweeteners. Unfortunately, in approximately 50% of the human population, steviol glycosides trigger the mouthfeel with a lingering bitter aftertaste. CarbExplore Research glucansucrase technology allowed glucosylation of the steviol glycosides rebaudioside A (RebA) and stevioside, especially when using Gtf180 mutant Q1140E. The product RebA-G is glucosylated only at the Glc(β1→C-19) residue, with the initial formation of an (α1→6) linkage. According to professional taste panels, the resulting product (a RebD analog) is significantly less bitter, resulting in an excellent taste profile.
Workflow:
You deliver:
Detailed project information about the product of interest.
We deliver:
A project plan with detailed descriptions.
We also provide the following analytical techniques:
Enhancing the solubility of surfactants
CarbExplore Research is also engaged in the glycosylation of surface-active agents (surfactants). These amphiphilic molecules with a hydrophobic and hydrophilic part form micelles in a solution. Surfactants have the characteristic feature to absorb in an air-water or oil-water interface, lowering the surface tension of a liquid. These cleaning solutions are widely used as detergents, emulsifiers, wetting agents etc.
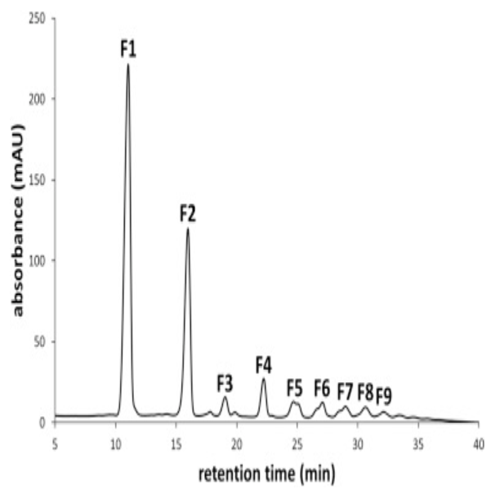
Gerwig J. et al. Carbohydrate research (2017) V 440-441 p 51-62. HPLC profile after a 24-h incubation of RebA with wild-type Gtf180-ΔN enzyme and sucrose at pH 4.7 and 37 °C.
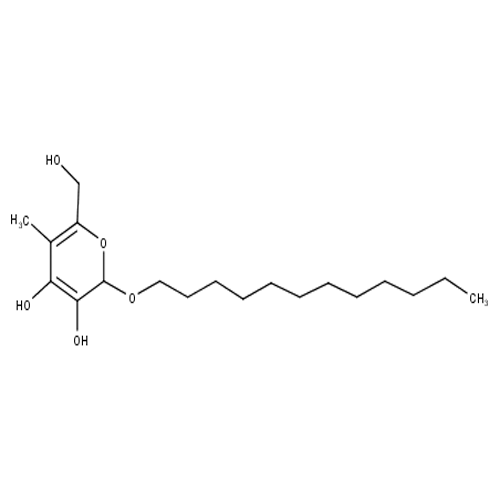
Molecule structure of an alkyl polyglycoside
Alkyl polyglucosides (APG) constitute a group of non-ionic surfactants which are used to enhance the formation of foam in a detergent. CarbExplore Research glucansucrase technology allowed glucosylation of various APGs. These APGs consist of a (poly) glucose head group and a hydrophobic alkyl chain. The attachment of a sugar molecule resulted in clear changes in the physicochemical properties of the APGs. Sugars contain numerous hydroxyl groups making them readily dissolvable in water due to the formation of hydrogen bonds. Glucosylation of these hydrophobic APG compounds thus yields potentially high value products.
Our Relevant Publications:
Publications 2019
Devlamynck, T. et al., 2019. Trans-α-glucosylation of stevioside by the mutant glucansucrase enzyme Gtf180-ΔN-Q1140E improves its taste profile. Food Chemistry, 30 1, Volume 272, pp. 653-662. DOI 10.1016/j.foodchem.2018.08.025
Pham, H. T., Boger, M. C., Dijkhuizen, L. & van Leeuwen, S. S., 2019. Stimulatory effects of novel glucosylated lactose derivatives GL34 on growth of selected gut bacteria. Applied Microbiology and Biotechnology, 18 1, 103(2), pp. 707-718. DOI 10.1007/s00253-018-9473-8
Pham, H. T., Ten Kate, G. A., Dijkhuizen, L. & Van Leeuwen, S. S., 2019. Synthesis and characterization of sialylated lactose- and lactulose-derived oligosaccharides by Trypanosoma cruzi trans-sialidase. Journal of Agricultural and Food Chemistry, 27 3, 67(12), pp. 3469-3479. DOI 10.1021/acs.jafc.8b06974
Publications 2018
Pham, H., Pijning, T., Dijkhuizen, L. & Van Leeuwen, S. S., 2018. Mutational analysis of the role of the glucansucrase Gtf180-ΔN active site residues in product and linkage specificity with lactose as acceptor substrate. Journal of Agricultural and Food Chemistry, 28 11, 66(47), pp. 12544-12554. DOI 10.1021/acs.jafc.8b04486
Pham, H. T., Dijkhuizen, L. & van Leeuwen, S. S., 2018. Structural characterization of glucosylated GOS derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydrate Research, 1 12, Volume 470, pp. 57-63. DOI 10.1016/j.carres.2018.10.003
Te Poele, E. M. et al., 2018. Glucansucrase (mutant) enzymes from Lactobacillus reuteri 180 efficiently transglucosylate Stevia component rebaudioside A, resulting in a superior taste. Scientific Reports, 1 12.8(1516). DOI 10.1038/s41598-018-19622-5
Publications 2017
Gerwig, G. J., te Poele, E. M., Dijkhuizen, L. & Kamerling, J. P., 2017. Structural analysis of rebaudioside A derivatives obtained by Lactobacillus reuteri 180 glucansucrase-catalyzed trans-α-glucosylation. Carbohydrate Research, Volume 440-441, pp. 51-62. DOI 10.1016/j.carres.2017.01.008
te Poele, E. M. et al., 2017. Catechol glucosides act as donor/acceptor substrates of glucansucrase enzymes of Lactobacillus reuteri. Applied Microbiology and Biotechnology, 1 6, 101(11), pp. 4495-4505. DOI 10.1007/s00253-017-8190-z
Pham, H. T., Dijkhuizen, L. & van Leeuwen, S. S., 2017. Structural characterization of glucosylated lactose derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydrate Research, Volume 449, pp. 59-64. DOI 10.1016/j.carres.2017.07.002
Publications 2016
Devlamynck, T. et al., 2016. Glucansucrase Gtf180-ΔN of Lactobacillus reuteri 180: enzyme and reaction engineering for improved glycosylation of non-carbohydrate molecules. Applied Microbiology and Biotechnology, 1 9, 100(17), pp. 7529-7539. DOI 10.1007/s00253-016-7476-x
Gerwig, G. J., te Poele, E. M., Dijkhuizen, L. & Kamerling, J. P., 2016. Stevia glycosides: chemical and enzymatic modifications of their carbohydrate moieties to improve the sweet-tasting quality. In: D. C. Baker, ed. Advances in Carbohydrate Chemistry and Biochemistry. s.l.:Academic Press, pp. 1-72. DOI 10.1016/bs.accb.2016.05.001
Te Poele, E. M., Grijpstra, P., Van Leeuwen, S. S. & Dijkhuizen, L., 2016. Glucosylation of catechol with the GTFA glucansucrase enzyme from Lactobacillus reuteri and sucrose as donor substrate. Bioconjugate Chemistry, 20 4, 27(4), pp. 937-946. DOI 10.1021/acs.bioconjchem.6b00018
Publications 2015
Publications 2014
Wilbrink, M. H. et al., 2014. Galactosyl-lactose sialylation using Trypanosoma cruzi trans-sialidase as the biocatalyst and bovine κ-casein-derived glycomacropeptide asthe donor substrate. Applied and Environmental Microbiology, 80(19), pp. 5984-5991. DOI 10.1128/AEM.01465-14
Publications 2013
Timm, M. et al., 2013. An unconventional glycosyl transfer reaction: Glucansucrase GTFA functions as an allosyltransferase enzyme. ChemBioChem, 12, 14(18), pp. 2423-2426. DOI 10.1002/cbic.201300392
Publications 2012
Desmet, T. et al., 2012. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chemistry – A European Journal, 27 8, 18(35), pp. 10786-10801. DOI 10.1002/chem.201103069