Nature provides an enormous variation in carbohydrate structures with numerous biological functions. Understanding the functional properties of carbohydrates on basis of their detailed structures is the Holy Grail in scientific research and industrial applications.
Elucidating the structure of an unknown carbohydrate compound is like solving a complicated puzzle. Carbohydrates may vary in monomer composition, e.g. glucose, fructose, and galactose. These building blocks are connected via glycosidic linkages, in an alpha or beta configuration, forming linear or branched chains of disaccharides, oligosaccharides, or polysaccharides. They may occur as free oligosaccharides, or be covalently linked as glycans to proteins, or lipids. Moreover, many plant metabolites constitute glycosylated non-carbohydrate compounds. Mixtures of oligosaccharides are also found in nature, e.g. in mammalian milk, or are produced commercially, e.g. the prebiotic compounds galacto- and fructo-oligosaccharides. Knowledge about the detailed composition of such mixtures is often crucial for (optimization of) their functional properties.
CarbExplore Research is able to perform the structural analysis of such carbohydrates. Our team uses the latest technology to address the questions of our clients. We have expertise in the structural analysis of all kinds of (mixtures of) carbohydrates, ranging from characterization of pure compounds to identification of unwanted process impurities.
CarbExplore offers several methods to analyze the carbohydrate target. The selection of the applied method(s) depends on our client’s questions, the provided samples and the level of information that is required. A summary of techniques employed:
- UPLC-UV/FLD (analytical and preparative mode) to separate and purify individual carbohydrates from mixtures
- HPAEC-PAD (analytical and preparative mode) to investigate the presence of mono-, di- and oligosaccharides and purify specific sugars from mixtures
- GC-MS analysis of partially methylated alditol acetate (PMAA) derivatives to determine the glycosidic linkages
- GC-MS analysis of trimethylsilyl (TMS)/alditol acetate (AA) derivatizatives to determine the monosaccharide composition
- 1D and 2D NMR experiments for structural elucidation of molecular structures and purity determination
- MALDI-TOF-MS and MS/MS for structural elucidation
- HP-SEC analysis, to determine chain length distribution and sizes of molecules
- Enzymatic digestion, to fractionate molecules into their building blocks and obtain further structural information.
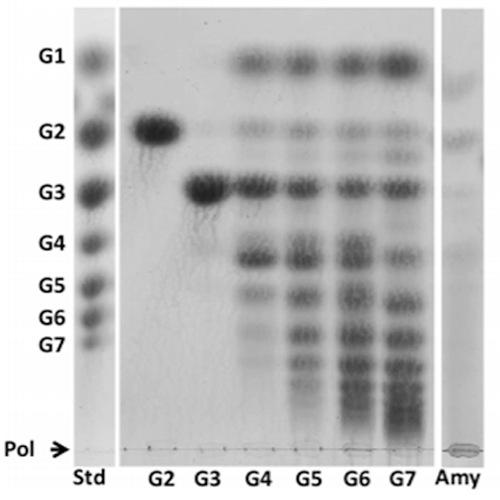
Gangoiti J. et al. (2016) Applied and Environmental Microbiology v. 82 no. 2. TLC analysis of the products synthesized by E. sibiricum 255-15 GtfC using MOS DP 2-7 and amylose.
We also provide the following analytical techniques:
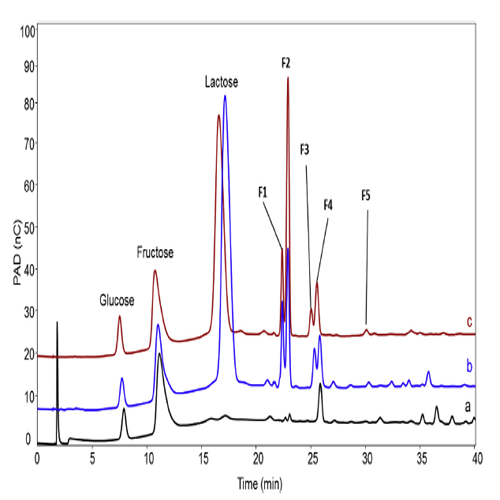
Pham H.T.T. et al. (2017) Carbohydrate Research 449: 59-64. HPAEC-PAD chromatograms of the reaction product mixtures obtained with (a) GtfA-ΔN with sucrose; (b) GtfA-ΔN with sucrose and lactose; and (c) Gtf180-ΔN with sucrose and lactose.
Our Relevant Publications:
Pubications 2020
te Poele, E. et al., 2020. Development of slowly digestible starch derived alpha-glucans with 4,6-α-glucanotransferase and branching sucrase enzymes. Journal of Agricultural and Food Chemistry, 21 5.p. acs.jafc.0c01465. DOI 10.1021/acs.jafc.0c01465
Valk-Weeber, R. L. et al., 2020. In depth analysis of the contribution of specific glycoproteins to the overall bovine whey N-linked glycoprofile. Journal of Agricultural and Food Chemistry, 21 5.p. acs.jafc.0c00959. DOI 10.1021/acs.jafc.0c00959
Kittibunchakul, S. et al., 2020. Structural comparison of different galacto-oligosaccharide mixtures formed by β-galactosidases from lactic acid bacteria and bifidobacteria. Journal of Agricultural and Food Chemistry, 15 4, 68(15), pp. 4437-4446. DOI 10.1021/acs.jafc.9b08156
Valk-Weeber, R., Eshuis-de Ruiter, T., Dijkhuizen, L. & van Leeuwen, S., 2020. Dynamic temporal variations in bovine lactoferrin glycan structures. J. Agric. Food Chem., 68(2), pp. 549-560. DOI 10.1021/acs.jafc.9b06762
Publications 2019
Böger, M., van Leeuwen, S., Lammerts van Bueren, A. & Dijkhuizen L., 2019. Structural identity of galactooligosaccharide molecules selectively utilized by single cultures of probiotic bacterial strains. J. Agric. Food Chem., 67(50), p. 13969–13977. DOI 10.1021/acs.jafc.9b05968
Devlamynck, T. et al., 2019. Trans-α-glucosylation of stevioside by the mutant glucansucrase enzyme Gtf180-ΔN-Q1140E improves its taste profile. Food Chemistry, 30 1, Volume 272, pp. 653-662. DOI 10.1016/j.foodchem.2018.08.025
Pham, H. T., Ten Kate, G. A., Dijkhuizen, L. & Van Leeuwen, S. S., 2019. Synthesis and characterization of sialylated lactose- and lactulose-derived oligosaccharides by Trypanosoma cruzi trans-sialidase. Journal of Agricultural and Food Chemistry, 27 3, 67(12), pp. 3469-3479. DOI 10.1021/acs.jafc.8b06974
Publications 2018
Boger, M. C. L., Lammerts van Bueren, A. & Dijkhuizen, L., 2018. Cross-feeding among probiotic bacterial strains on prebiotic inulin involves the extracellular exo-inulinase of Lactobacillus paracasei strain W20. Applied and Environmental Microbiology, 1 11, 84(21), pp. e01539-18. DOI 10.1128/AEM.01539-18
van Leeuwen, S. S. et al., 2018. Regional variations in human milk oligosaccharides in Vietnam suggest FucTx activity besides FucT2 and FucT3. Scientific Reports, 1 12.8(16790). DOI 10.1038/s41598-018-34882-x
Pham, H. T., Dijkhuizen, L. & van Leeuwen, S. S., 2018. Structural characterization of glucosylated GOS derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydrate Research, 1 12, Volume 470, pp. 57-63. DOI 10.1016/j.carres.2018.10.003
Te Poele, E. M. et al., 2018. Glucansucrase (mutant) enzymes from Lactobacillus reuteri 180 efficiently transglucosylate Stevia component rebaudioside A, resulting in a superior taste. Scientific Reports, 1 12.8(1516). DOI 10.1038/s41598-018-19622-5
Yin, H., Dijkhuizen, L. & van Leeuwen, S. S., 2018. Synthesis of galacto-oligosaccharides derived from lactulose by wild-type and mutant β-galactosidase enzymes from Bacillus circulans ATCC 31382. Carbohydrate Research, 30 7, Volume 465, pp. 58-65. DOI 10.1016/j.carres.2018.06.009
Publications 2017
Gangoiti, J. et al., 2017. 4,3-α-Glucanotransferase, a novel reaction specificity in glycoside hydrolase family 70 and clan GH-H. Scientific Reports, 6 1.7(39761). DOI 10.1038/srep39761
Pham, H. T., Dijkhuizen, L. & van Leeuwen, S. S., 2017. Structural characterization of glucosylated lactose derivatives synthesized by the Lactobacillus reuteri GtfA and Gtf180 glucansucrase enzymes. Carbohydrate Research, Volume 449, pp. 59-64. DOI 10.1016/j.carres.2017.07.002
Gangoiti, J. et al., 2017. Mining novel starch-converting Glycoside Hydrolase 70 enzymes from the Nestlé culture collection genome database: the Lactobacillus reuteri NCC 2613 GtfB. Scientific Reports, 1 12.7(9947). DOI 10.1038/s41598-017-07190-z
Yin, H., Bultema, J. B., Dijkhuizen, L. & van Leeuwen, S. S., 2017. Reaction kinetics and galactooligosaccharide product profiles of the β-galactosidases from Bacillus circulans, Kluyveromyces lactis and Aspergillus oryzae. Food Chemistry, 15 6, Volume 225, pp. 230-238. DOI 10.1016/j.foodchem.2017.01.030
Gerwig, G. J., te Poele, E. M., Dijkhuizen, L. & Kamerling, J. P., 2017. Structural analysis of rebaudioside A derivatives obtained by Lactobacillus reuteri 180 glucansucrase-catalyzed trans-α-glucosylation. Carbohydrate Research, Volume 440-441, pp. 51-62. DOI 10.1016/j.carres.2017.01.008
Publications 2016
Gerwig, G. J., te Poele, E. M., Dijkhuizen, L. & Kamerling, J. P., 2016. Stevia glycosides: chemical and enzymatic modifications of their carbohydrate moieties to improve the sweet-tasting quality. In: D. C. Baker, ed. Advances in Carbohydrate Chemistry and Biochemistry. s.l.:Academic Press, pp. 1-72. DOI 10.1016/bs.accb.2016.05.001
van Leeuwen, S. S., Kuipers, B. J., Dijkhuizen, L. & Kamerling, J. P., 2016. Comparative structural characterization of 7 commercial galacto-oligosaccharide (GOS) products. Carbohydrate Research, 29 4, Volume 425, pp. 48-58. DOI 10.1016/j.carres.2016.03.006
Meng, X. et al., 2016. Synthesis of new hyperbranched α-Glucans from Sucrose by Lactobacillus reuteri 180 glucansucrase mutants. Journal of Agricultural and Food Chemistry, 20 1, 64(2), pp. 433-442. DOI 10.1021/acs.jafc.5b05161
Publications 2015
Meng, X. et al., 2015. Synthesis of oligo- and polysaccharides by Lactobacillus reuteri 121 reuteransucrase at high concentrations of sucrose. Carbohydrate Research, 23 9, Volume 414, pp. 85-92. DOI 10.1016/j.carres.2015.07.011
Publications 2014
Leemhuis, H. et al., 2014. Isomalto/malto-polysaccharide, a novel soluble dietary fiber made via enzymatic conversion of starch. Journal of Agricultural and Food Chemistry, 10 12, 62(49), pp. 12034-12044. DOI 10.1021/jf503970a
Van Leeuwen, S. S., Kuipers, B. J., Dijkhuizen, L. & Kamerling, J. P., 2014. 1H NMR analysis of the lactose/β-galactosidase-derived galacto-oligosaccharide components of Vivinal® GOS up to DP5. Carbohydrate Research, 5 12, Volume 400, pp. 59-73. DOI 10.1016/j.carres.2014.08.012
Van Leeuwen, S. S., Kuipers, B. J., Dijkhuizen, L. & Kamerling, J. P., 2014. Development of a 1H NMR structural-reporter-group concept for the analysis of prebiotic galacto-oligosaccharides of the [β-d-Galp-(1→x)]n-d-Glcp type. Carbohydrate Research, 5 12, Volume 400, pp. 54-58. DOI 10.1016/j.carres.2014.08.011
Wilbrink, M. H. et al., 2014. Galactosyl-lactose sialylation using Trypanosoma cruzi trans-sialidase as the biocatalyst and bovine κ-casein-derived glycomacropeptide asthe donor substrate. Applied and Environmental Microbiology, 80(19), pp. 5984-5991. DOI 10.1128/AEM.01465-14
Publications 2013
Dobruchowska, J. M. et al., 2013. Gluco-oligomers initially formed by the reuteransucrase enzyme of Lactobacillus reuteri 121 incubated with sucrose and malto-oligosaccharides. Glycobiology, 9, 23(9), pp. 1084-1096. DOI 10.1093/glycob/cwt048