BgaD-D of Bacillus circulans ATCC 31382
Request quote for price
Beschrijving
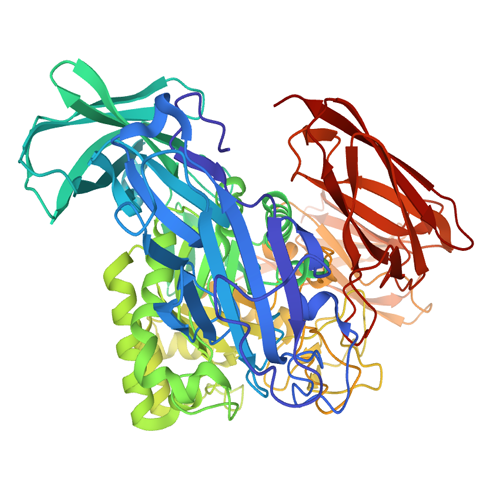
The C-terminally truncated β-galactosidase enzyme from Bacillus circulans ATCC 31382 is a member of Glycoside Hydrolase family 2. This enzyme, also known as BgaD-D, is the shortest isoform of the full-length protein BgaD. The microbial enzyme is able to synthesize prebiotic galactooligosaccharides (GOS) and Oligos from lactulose (OsLu). These products are widely used as biocatalyst in the food industry, because of their healthy effect on humans. GOS mixtures mimic the prebiotic effects of the human milk oligosaccharides, and therefore are added to infant formula.
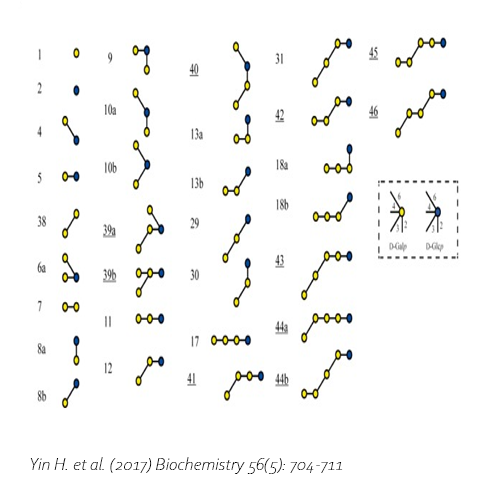
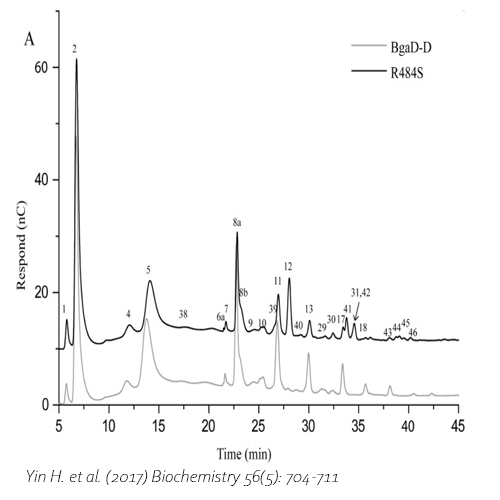
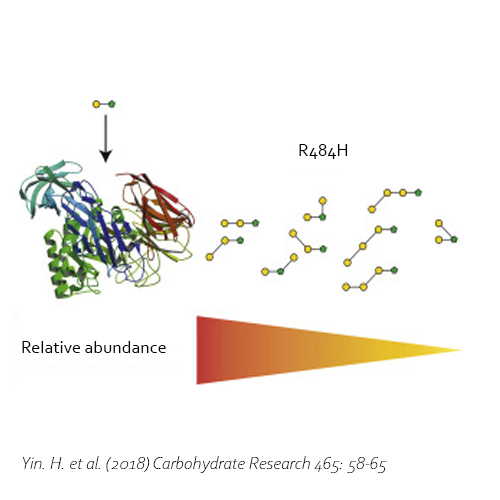
Compared with commercial available β-galactosidase enzymes, the BgaD-D enzyme of B. circulans generates a high yield of GOS showing a complex formation pattern. The GOS product synthesized by the BgaD-D protein presents a carbohydrate mixture which contains a majority of β(1→4) linked galactose on the reducing glucose residue, and a trace amount of β(1→2), β(1→3), β(1→6) linkages. The products contain both linear and branched structures. Some structures have been further elongated with β(1→4)-linked galactose residues.
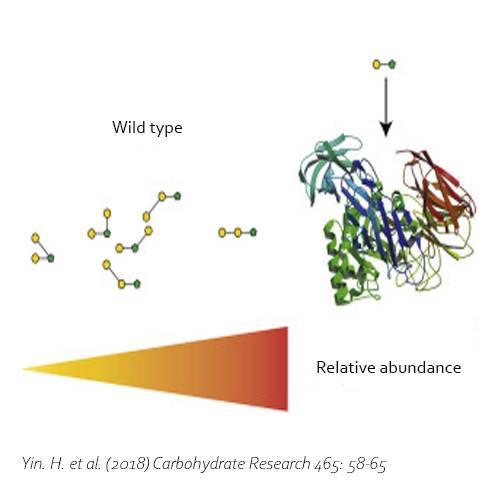
Moreover, the BgaD-D enzyme is able to synthesize oligosaccharides out of lactulose (OsLu). Although the yields are rather low compared with GOS production using lactose, the enzyme creates high-value compounds. The products of this β-galactosidase have a preference for introducing β(1→4) and β(1→3) linkages. OsLu may act as prebiotic compounds and are potentially resistant to gut digestion.
Human newborns are capable to produce β-galactosidase enzymes to digest lactose. Nevertheless, the majority of adults have lost the ability to produce these enzymes. This can end up in lactose intolerance, making them incapable to digest dairy products. The lactose could be removed from the milk to solve this problem, and the residual product could be converted to high valuable lactose derivates like GOS.
Yin, H., Dijkhuizen, L. & van Leeuwen, S. S., 2018. Synthesis of galacto-oligosaccharides derived from lactulose by wild-type and mutant β-galactosidase enzymes from Bacillus circulans ATCC 31382. Carbohydrate Research, 30 7, Volume 465, pp. 58-65. DOI 10.1016/j.carres.2018.06.009
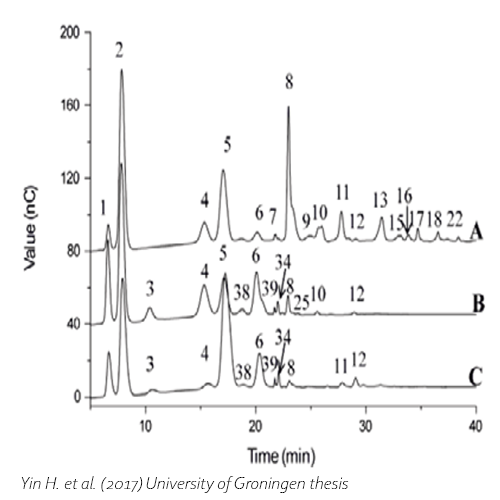
Yin, H., 2017. Biochemical characterization of β-galactosidases and engineering of their product specificity, University of Groningen.
Yin, H. et al., 2017. Engineering of the Bacillus circulans β-galactosidase product specificity. Biochemistry, 7 2, 56(5), pp. 704-711. DOI 10.1021/acs.biochem.7b00032
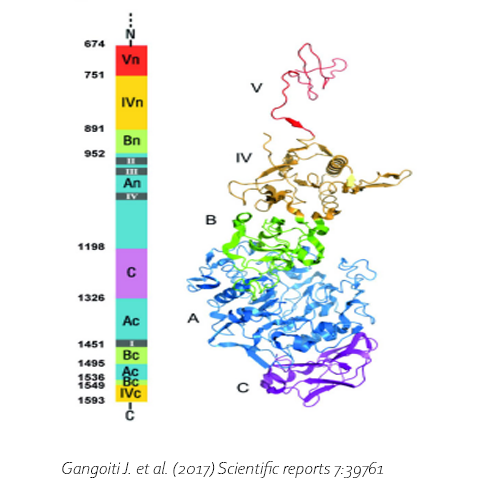
Yin H., Pijning T., Meng X., Dijkhuizen L., van Leeuwen S.S. (2017) Biochemical characterization of the functional roles of residues in the active site of the β-galactosidase from Bacillus circulans ATCC 31382. Biochemistry 56: 3109−3118
Bultema, J. B., Kuipers, B. J. & Dijkhuizen, L., 2014. Biochemical characterization of mutants in the active site residues of the β-galactosidase enzyme of Bacillus circulans ATCC 31382. FEBS Open Bio, 1 12, Volume 4, pp. 1015-1020.
Extra informatie
| Volume | 1000 units, 300 units |
|---|