GtfD 4,6-α-glucanotransferase of Azotobacter chroococcum
Request quote for price
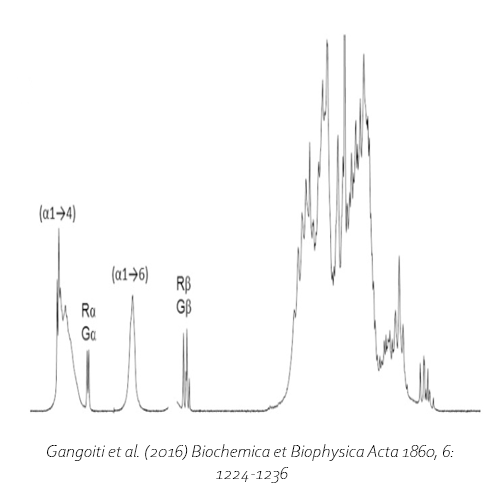
The GtfD enzyme of the gram-negative bacteria Azotobacter chroococcum is a unique evolutionary intermediate between glycoside hydrolase family GH13 (α-amylase) and GH70 (glucansucrase). The enzyme displays 4,6-α-glucanotransferase activity using malto-oligosaccharides (> DP 4), and starch. However, the enzyme is unable to synthesize consecutive α(1→6) glucosidic bonds. Instead it forms a high molecular mass α(1→4,6) branched reuteran like polymer with 68 % α(1→4), and 32 % α(1→6) glycosidic linkages using amylose V as substrate. The glucan is highly similar to the product formed by Lactobacillus reuteri GtfA glucansucrase from sucrose. The products formed by the GtfD enzyme is potentially useful for the conversion of starch in the food industry.
This product is sold for research use only.
*Enzyme activity was determined by the iodine-staining assay using amylose as substrate. One unit of activity is defined as the amount of enzyme preparation converting 1 mg of substrate per min.